Table of Contents
Home / Use Case /
AI in Drug Development: Applications, Benefits, Challenges, and What’s Next

AI in drug development is transforming the process of discovering and bringing new medicines to market. By leveraging machine learning and deep neural networks, pharmaceutical companies can now process massive biological datasets, model molecular interactions, and identify viable drug candidates much faster than traditional approaches.
Bringing a drug to market has always been time-consuming and costly. According to analyses of FDA approvals from 2009 to 2018, the median capitalised R&D cost to develop a new drug was estimated at $985 million, with the mean exceeding $1.3 billion. AI is helping to cut down those figures by accelerating critical R&D stages, especially in early discovery and screening.
AI Adoption is widespread in the health sector. As of early 2024, nearly 80% of pharmaceutical and life sciences professionals reported actively using AI in drug discovery workflows.
One study involving AI-accelerated virtual Screening found hit rates of 14% and 44% for two targets, successfully identifying promising compounds from multi-billion-molecule libraries in under 7 days. This demonstrates how AI dramatically accelerates and enhances the early-stage drug discovery process, a cornerstone of modern drug development.
Major breakthroughs—like AlphaFold’s protein folding predictions or generative AI in de novo molecule design—are pushing boundaries. At the same time, AI tools are helping personalise treatments, identify rare disease pathways, and reduce trial failures. Regulatory bodies, such as the FDA, are also developing frameworks to accommodate AI-driven models in clinical validation processes.
With AI revolutionising the way drugs are discovered, developed, approved, and delivered, its integration isn’t just a tech trend, but also quickly becoming an industry standard.
Let AI Power Your Next Breakthrough
Use machine learning to uncover targets, optimise compounds, and streamline trials—smarter, faster, and with higher success rates.
History of AI in Drug Discovery and Drug Development
Artificial intelligence began making its mark in pharmaceutical research during the 1980s, primarily through computational chemistry and cheminformatics for drug design.
By the early 2000s, advances in bioinformatics, genomics, and computing power enabled AI tools to assist with target identification and compound screening. The real acceleration came post-2015 with the rise of deep learning and cloud-based analytics.
In 2020, Exscientia’s AI-designed molecule DSP-1181 entered human trials—a major breakthrough. AI now spans all stages, from early discovery to clinical trial optimisation. Its evolution reflects a shift from rule-based systems to predictive, adaptive models that integrate genomics, real-world data, and multi-omics. The history of AI in drug discovery is marked by gradual integration, culminating in its central role today.
Overview of AI in Drug Discovery and Development
AI plays a pivotal role in reshaping pharmaceutical R&D, offering scalable, data-driven insights throughout the drug development pipeline. It aids researchers in understanding complex biological systems, predicting drug efficacy, and minimising late-stage failures.
AI tools sift through massive biomedical databases, simulate molecule-target interactions, and model clinical outcomes—all faster and more accurately than traditional methods. Pharmaceutical companies now use platforms powered by deep learning, reinforcement learning, and generative models to automate hit discovery, de-risk lead optimisation, and improve trial designs.
With the increasing availability of multi-omics datasets and real-world evidence, AI is not only supporting decisions but also guiding them. This shift enhances efficiency, reduces costs, and paves the way for faster, safer therapies to reach patients globally.
Businesses looking to implement such advanced AI infrastructure often hire AI developers who specialise in machine learning, bioinformatics, and predictive analytics to tailor systems for their therapeutic focus.
Benefits of AI in Drug Discovery and Development
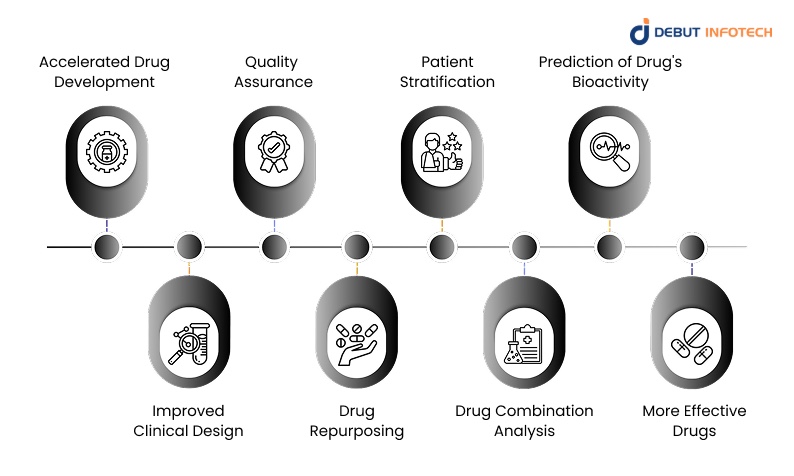
The integration of AI offers significant advantages, including faster timelines, lower costs, and higher accuracy. Here are the core benefits that make AI an indispensable tool in modern drug development.
1. Accelerated Drug Development
AI-driven models streamline various stages of the drug pipeline, significantly reducing the time required for target identification, compound screening, and lead optimisation.
By rapidly filtering out low-potential candidates through simulations and virtual screening, AI enables researchers to focus only on viable compounds. This approach compresses development timelines from over a decade to just a few years in some cases, saving both time and resources.
2. Improved Clinical Design
Clinical trials are often delayed by poor design and participant mismatch. AI leverages patient data, biomarkers, and predictive analytics to optimise trial protocols.
It helps researchers identify eligible cohorts more precisely and forecast trial outcomes. This improves recruitment, reduces costs, and increases the chance of success—ultimately improving the clinical development process across therapeutic areas.
3. Quality Assurance
Quality assurance is critical in both preclinical research and production. AI systems automate anomaly detection and monitor laboratory data for inconsistencies. They can identify deviations in real-time and flag potential risks before they escalate.
By integrating historical trends with real-time inputs, AI helps maintain regulatory compliance and ensure product integrity throughout the entire product lifecycle, from development to post-marketing.
4. Drug Repurposing
AI enhances drug repurposing by comparing known drugs against vast datasets of diseases, symptoms, and molecular pathways.
By identifying unexpected drug-disease relationships, AI allows researchers to explore new indications for approved drugs. This not only reduces the risk of failure but also accelerates time-to-market, as these drugs already meet many safety requirements.
5. Patient Stratification
AI assists in patient stratification by analysing genomic, clinical, and lifestyle data to group patients according to likely treatment response. This improves the precision of clinical trials and ensures that therapies are tested on the right populations. The result is more effective outcomes, lower dropout rates, and stronger data for regulatory approval.
6. Drug Combination Analysis
Determining effective drug combinations is complex due to potential interactions. AI simulates combination therapies and models synergistic or antagonistic effects across large datasets. These insights help researchers discover novel combinations that maximise efficacy while minimising toxicity—particularly valuable in cancer and infectious disease treatment where multi-drug regimens are common.
7. Prediction of Drug’s Bioactivity
AI models predict a compound’s biological activity based on its structure and the biological activity of its historical analogues. This enables researchers to predict how a drug will interact with its targets before any laboratory work begins. Techniques such as molecular docking, QSAR models, and neural networks reduce the dependency on time-consuming experiments, saving both cost and effort.
8. More Effective Drugs
AI increases the probability of discovering drugs with higher therapeutic efficacy. By integrating data on target biology, pharmacodynamics, and patient profiles, AI can propose molecules tailored for optimal interaction and minimal side effects. This precision-driven approach enhances clinical success rates and facilitates the development of safer, more effective medications.
Cut Development Costs Without Cutting Corners
Leverage automation and prediction models to reduce trial-and-error, lower spending, and increase R&D efficiency.
AI Techniques in Drug Discovery
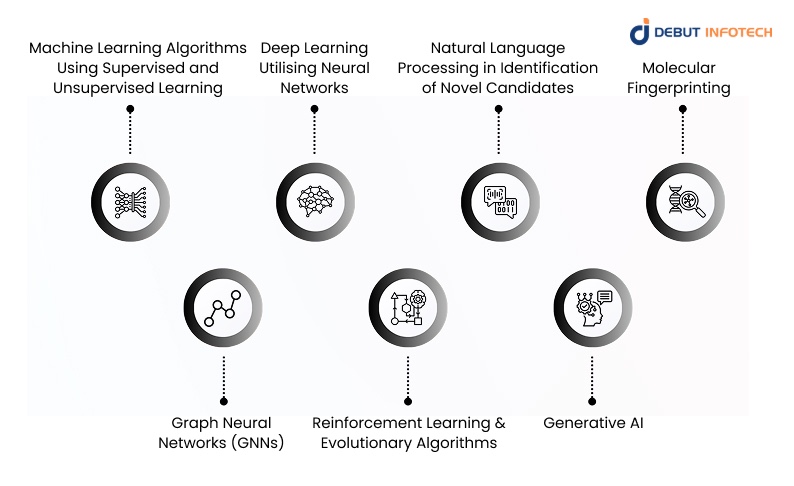
1. Machine Learning Algorithms Using Supervised and Unsupervised Learning
Machine learning (ML) plays a foundational role in drug discovery by enabling the recognition of patterns and predictive modelling from vast biomedical datasets. Supervised learning models are trained on labelled data to classify compounds, predict toxicity, or assess efficacy.
In contrast, unsupervised learning uncovers hidden structures or clusters in unlabeled datasets, aiding in the identification of novel biological targets. These models analyse clinical outcomes, omics data, and molecular interactions to generate insights at unprecedented speed and accuracy.
As more validated data becomes available, ML algorithms become more robust and transferable, supporting scalable innovation in hit identification, lead optimisation, and preclinical research phases.
2. Deep Learning Utilising Neural Networks
Deep learning uses multilayered neural networks to simulate complex relationships between molecular structures, biological systems, and pharmacological outcomes. These networks, including convolutional neural networks (CNNs) and recurrent neural networks (RNNs), are adept at processing high-dimensional data, such as protein structures or 3D molecular conformations.
Deep learning enables high-precision virtual screening, protein folding predictions, and ligand-binding affinity modelling. As datasets grow in size and complexity, deep learning systems improve by learning non-linear patterns that traditional models might miss. This capability allows researchers to make more informed decisions, accelerate compound optimisation, and reduce late-stage clinical failure caused by poor molecular performance predictions.
3. Natural Language Processing in Identification of Novel Candidates
Natural Language Processing (NLP) enables AI to read and understand scientific literature, patents, trial registries, and clinical reports. By scanning millions of documents in real-time, NLP tools extract relevant entities—such as drug names, gene targets, and disease links—and synthesise this information to generate novel drug hypotheses. This significantly reduces manual review effort and ensures that no critical findings are overlooked.
Platforms using NLP have helped identify previously unconsidered drug-disease relationships and predict mechanism-of-action profiles by integrating cross-domain knowledge. The speed and scale at which NLP operates make it an indispensable technique in the early-stage identification of drug candidates and biomarker discovery.
4. Molecular Fingerprinting
Molecular fingerprinting transforms the chemical structure of compounds into digital vectors or bit strings that represent their physicochemical and structural properties. These fingerprints enable the rapid comparison of large compound libraries, allowing algorithms to identify compounds with similar pharmacological properties.
Commonly used fingerprints include ECFP (Extended Connectivity Fingerprints) and MACCS keys. Fingerprinting supports virtual screening, lead optimisation, and SAR (Structure-Activity Relationship) analysis by flagging molecular substructures associated with desired activity or toxicity.
When integrated with machine learning models, these representations become even more powerful—enhancing compound prioritisation, reducing redundant testing, and expediting the path from discovery to candidate selection.
5. Graph Neural Networks (GNNs)
Graph Neural Networks represent molecules as graphs, where atoms are represented as nodes and bonds are represented as edges, enabling AI to understand the spatial and relational structure of compounds more effectively.
Unlike linear descriptors, GNNs retain topological information, capturing both local and global interactions. These networks can learn to predict molecular properties, such as solubility, bioavailability, and target binding affinity.
In drug discovery, GNNs have shown success in toxicity prediction, reaction outcome modelling, and compound similarity analysis. Their ability to process complex molecular structures makes them ideal for predicting interactions in dynamic biological environments. This ultimately improves the accuracy and interpretability of drug screening processes.
6. Reinforcement Learning and Evolutionary Algorithms
Reinforcement learning (RL) and evolutionary algorithms are optimisation techniques that generate and refine molecular candidates through trial-and-error simulations. RL models are trained to explore chemical space and receive rewards for producing drug-like, bioactive, or non-toxic compounds.
Evolutionary algorithms mimic natural selection by combining and mutating successful molecules across generations to evolve more effective drug structures.
Both methods can navigate complex, high-dimensional drug design challenges that traditional rule-based approaches often fail to address. These models help discover novel scaffolds and optimise ADMET (absorption, distribution, metabolism, excretion, and toxicity) profiles—making them especially valuable in lead optimisation and de novo drug design efforts.
7. Generative AI
Generative AI, including Generative Adversarial Networks (GANs) and transformer-based models, creates novel molecular structures with desired properties by learning from vast chemical databases. These models generate de novo compounds that meet predefined biological or physicochemical criteria, such as target binding affinity or metabolic stability.
By simulating chemical reactions and structural variations, generative AI reduces reliance on traditional synthesis and broadens the scope of discovery. Companies now use these tools to explore uncharted areas of chemical space and address diseases with limited existing treatment options.
With continuous learning capabilities, generative AI systems improve over time, transforming early-stage discovery and reducing R&D bottlenecks.
Related Read: Top Healthcare Software Development Companies
Use Cases of AI in Drug Development
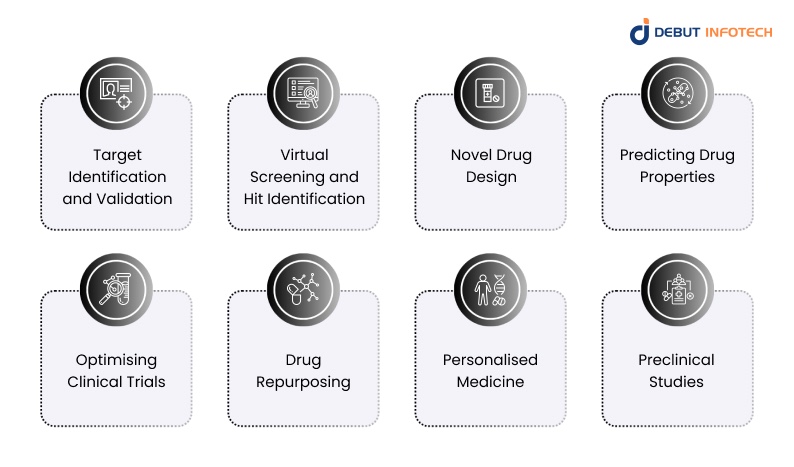
AI use cases in drug discovery and development are vast and expanding. Here are the key ways AI is being used in medicine to identify new molecules, predict their behaviour, and accelerate the R&D pipeline:
1. Target Identification and Validation
AI accelerates the early stage of drug development by identifying potential biological targets—such as proteins, genes, or pathways involved in a disease—and validating their relevance. By analysing genomics, proteomics, and disease network data, AI models uncover previously unknown or poorly understood targets. These platforms utilise predictive modelling and pattern recognition to link disease phenotypes with underlying biological mechanisms.
The accuracy and speed of AI reduce wasted resources spent on non-viable targets. This capability is especially valuable in oncology, neurology, and rare disease research, where conventional target identification methods often fall short. Validating these targets using AI also enhances downstream success rates, creating a more focused and efficient drug discovery pipeline.
Real-life example:
BenevolentAI used AI to identify a novel target for treating amyotrophic lateral sclerosis (ALS) by mining scientific literature, clinical data, and biological pathways.
The AI system linked an underexplored gene with ALS pathophysiology—previously overlooked by conventional research. This finding laid the foundation for a new therapeutic development program. It highlighted AI’s potential to uncover novel targets in complex diseases.
2. Virtual Screening and Hit Identification
Virtual screening is the computational process of rapidly assessing large libraries of compounds to identify potential “hits” that may interact with a biological target. AI enhances this process by using models trained to predict binding affinity, molecular interactions, and toxicity profiles. These platforms drastically reduce the number of compounds that need to be tested in vitro, thereby saving time and resources.
By integrating chemical fingerprints, 3D structure predictions, and ML classifiers, AI increases screening precision. It also allows researchers to prioritise compounds with favourable ADMET properties from the outset. Virtual screening, empowered by AI, significantly shortens the timeline from compound identification to lead optimisation.
Real-life example:
Atomwise applied its AI platform to screen over 10 million compounds for inhibitors of the Ebola virus. Within days, the system identified promising candidates, two of which were later validated to block the virus’s entry into host cells. This speed and accuracy would have been impossible with traditional screening methods, showcasing the power of AI in hit identification.
3. Novel Drug Design
Artificial intelligence facilitates de novo drug design by generating new molecular structures optimised for specific biological and chemical properties. Generative algorithms simulate interactions between potential compounds and their targets, while reinforcement learning guides the model toward drug-like molecules. These tools not only accelerate lead generation but also explore untapped chemical space, offering novel scaffolds that traditional approaches might never have discovered.
AI-driven design allows for multi-objective optimisation, balancing potency, safety, and manufacturability. This iterative, data-driven process improves the quality of drug candidates, reduces reliance on historical data, and enables innovation in disease areas with limited existing therapies.
Real-life example:
Insilico Medicine utilised AI to design a fibrosis-targeting molecule, which progressed from target selection to a preclinical candidate in under 18 months. The generative AI system produced novel compounds, evaluated them in silico, and refined them through feedback loops—demonstrating how AI can significantly shorten the early discovery timeline while delivering high-quality leads.
4. Predicting Drug Properties
Predicting a drug’s pharmacokinetic and pharmacodynamic properties is essential to determining its viability. AI excels in this domain by forecasting absorption, distribution, metabolism, excretion, and toxicity (ADMET) profiles. These predictions are based on molecular structure, known analogues, and previous experimental data. By flagging compounds with undesirable properties early, AI prevents costly failures during later stages of development.
Moreover, models can simulate how different patient populations might respond to a compound—refining dosage levels or formulations. This predictive capability enhances safety, minimises trial-and-error testing, and improves the efficiency of drug development across a wide range of therapeutic areas.
Real-life example:
AstraZeneca partnered with BenevolentAI to predict the cardiotoxicity and metabolic stability of new compounds. The AI model identified molecules with low predicted risk profiles, helping the team prioritise safer candidates for progression into clinical studies—streamlining risk assessment and accelerating preclinical validation.
5. Optimising Clinical Trials
AI transforms clinical trial design and execution by improving patient recruitment, refining endpoints, and simulating outcomes. Machine learning algorithms match patients to trials based on genetic markers, electronic health records, and eligibility criteria, thereby enhancing recruitment efficiency and trial diversity.
Predictive analytics can also model trial outcomes, allowing adaptive designs that respond to interim results. This reduces the number of required participants and improves the likelihood of reaching statistically significant results.
Moreover, AI detects early signals of adverse effects or treatment success, allowing better decision-making and real-time trial adjustments. These advancements lead to faster and more cost-effective trials, thereby increasing regulatory confidence.
Real-life example:
Pfizer used AI algorithms to optimize patient recruitment in oncology trials, identifying optimal trial sites and target demographics. This shortened enrollment periods and reduced dropout rates. The AI-driven approach increased trial efficiency and significantly lowered costs compared to traditional recruitment strategies.
6. Drug Repurposing
AI accelerates drug repurposing by analysing existing compounds and identifying new therapeutic indications. Through deep learning and graph-based models, AI evaluates relationships between drugs, diseases, pathways, and patient data to uncover potential overlaps. This method is especially valuable in urgent situations, such as pandemics, where time is limited and safety data from existing drugs can expedite development.
AI reduces the cost and time associated with developing drugs from scratch by leveraging prior approvals and known safety profiles. This makes drug repurposing a viable solution for rare or neglected diseases where traditional research has been limited.
Real-life example:
During the COVID-19 pandemic, BenevolentAI identified baricitinib—an approved medication for rheumatoid arthritis—as a potential treatment for COVID-19. Within weeks, clinical trials were initiated, and the drug was later granted emergency use authorisation. The AI-driven insight played a critical role in providing a timely therapeutic response.
7. Personalised Medicine
AI supports the shift toward personalised medicine by integrating genomic, epigenomic, clinical, and lifestyle data to tailor treatments to individual patients. This data fusion enables predictive modelling of drug responses, allowing clinicians to select the most effective therapies while minimising side effects.
AI can identify biomarkers for stratification and predict outcomes based on genetic variations. This approach enhances therapeutic efficacy and reduces the need for trial-and-error prescribing.
Personalised medicine is especially impactful in oncology and rare diseases, where precision in treatment selection significantly affects patient outcomes. AI ensures treatment decisions are data-informed, patient-specific, and adaptive to real-time health indicators.
Real-life example:
Tempus uses AI-powered platforms to match cancer patients with the most appropriate therapies based on their genomic profiles. In one case, the platform recommended an off-label treatment that led to a positive patient response—demonstrating the practical value of AI in personalised oncology care.
8. Preclinical Studies
AI enhances preclinical studies by predicting toxicological profiles, simulating in vivo responses, and identifying optimal dosage levels. Models trained on historical biological data can simulate organ-specific effects and predict the safety of compounds without the need for extensive animal testing. This leads to better-informed compound selection, reduces ethical concerns, and saves time and costs.
AI also aids in identifying relevant biomarkers and endpoints that can be carried into human trials. By narrowing down viable candidates before moving into expensive and time-intensive clinical phases, AI streamlines the preclinical workflow and improves overall R&D efficiency.
Real-life example:
IBM Watson and Sanofi collaborated to use AI for predicting hepatotoxicity in preclinical candidates. The system identified liver safety risks based on molecular features and previous toxicology data, enabling researchers to eliminate high-risk compounds before animal testing—saving months of lab work and improving the efficiency of their preclinical pipeline.
Build a Custom AI Solution for Your Pipeline
We tailor AI platforms to your specific drug development needs—scalable, secure, and ready to deploy.
Real-Life Impact of AI-Powered Drug Discovery and Development
1. MEK Protein Inhibitor Identification
AI has significantly accelerated the identification of MEK inhibitors, which are critical in cancer therapy. Using deep learning algorithms, researchers reduced the discovery time from years to mere months. Companies like BenevolentAI and Atomwise used predictive models to identify candidate molecules with high binding affinity and favourable bioavailability, cutting early-stage R&D costs and timelines drastically.
2. Cancer Treatment Compound Discovery
Insilico Medicine used generative adversarial networks (GANs) to design novel anti-cancer molecules, achieving promising preclinical outcomes. Their AI engine analysed massive molecular datasets to identify structures likely to inhibit cancer growth, leading to faster compound development and higher efficacy in lab models. This reflects how AI is transforming oncology pipelines globally.
3. Alzheimer’s Disease Therapeutic Targeting
AI models, such as IBM Watson and DeepMind’s AlphaFold, have enabled the identification of previously unknown protein targets associated with Alzheimer’s. These discoveries are helping pharmaceutical companies design more targeted treatments, which may potentially delay disease progression. It represents a significant advancement in one of the most complex neurological domains.
4. Novel Antibiotic Discovery
MIT researchers used AI to identify Halicin, a novel antibiotic effective against multidrug-resistant bacteria. The model screened over 100 million chemical compounds, selecting candidates that conventional methods had overlooked. This breakthrough highlights AI’s capacity to fight antibiotic resistance—a growing global health threat.
5. COVID-19 Therapeutic Research
During the pandemic, AI platforms such as Exscientia and BenevolentAI played a pivotal role in repurposing existing drugs and identifying new antiviral candidates. By mining biomedical literature and protein interaction maps, AI helped identify baricitinib as a potential treatment for COVID-19, which later received emergency use authorisation. It demonstrated how AI can respond swiftly during global health crises.
The Role of AI for Drug Discovery In Post-market Safety
1. Signal Detection
AI tools monitor electronic health records, social media, and real-world evidence to detect adverse drug reactions earlier than traditional methods. Machine learning models analyse large-scale data streams for unusual patterns that may indicate safety issues, enabling faster regulatory responses and enhanced patient safety.
2. Real-Time Monitoring
Real-time pharmacovigilance systems, powered by AI, track patient outcomes and medication effects continuously after market launch. This enables pharmaceutical firms and regulatory bodies to identify issues early and take proactive steps. Such systems help bridge the gap between clinical trial findings and real-world efficacy and safety.
3. Risk Prediction
By using AI models trained on clinical data, demographics, and genetic profiles, developers or AI development companies can predict potential adverse outcomes and tailor risk mitigation strategies. These models provide a probabilistic assessment of a drug’s long-term effects, improving confidence in post-market surveillance and guiding physician decision-making.
4. Drug-Drug Interactions
AI algorithms assess massive pharmacological datasets to detect and predict harmful drug-drug interactions. These systems evaluate biochemical mechanisms, dosage schedules, and patient-specific factors to ensure safer polypharmacy. Integrating these tools into electronic prescribing systems enhances clinical decision support and reduces medical errors.
Challenges of AI in Drug Development
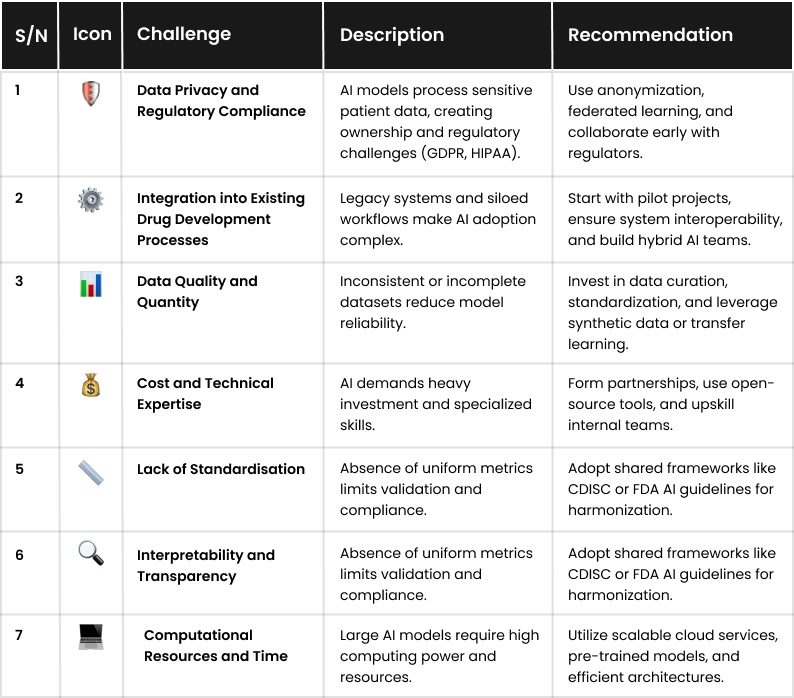
Despite its potential, AI in drug development faces several practical and ethical challenges. Each issue must be carefully addressed to ensure the technology delivers safe, effective, and equitable solutions. Here they are, as well as recommendations:
1. Data Privacy and Regulatory Compliance
AI systems often process sensitive patient data, raising concerns about data ownership, consent, and cross-border regulations such as GDPR or HIPAA. Pharmaceutical companies must comply with strict privacy laws while leveraging patient-level data, which can hinder the training of large-scale AI models. These legal constraints may limit collaboration, data sharing, or model scalability, especially in multinational projects involving multiple clinical datasets.
Recommendation:
Companies should adopt robust anonymisation techniques and use privacy-preserving AI methods such as federated learning or differential privacy. Collaborating with regulators early in the development cycle ensures clarity on acceptable AI practices and guidelines.
Clear data governance policies and transparent auditing protocols can also bridge trust gaps and support the development of secure, compliant AI applications in drug development.
2. Integration into Existing Drug Development Processes
AI solutions often remain siloed from conventional workflows, making integration into traditional research and clinical development pipelines challenging. Legacy systems, rigid protocols, and organisational resistance to new technologies can delay adoption or limit the transformative potential of AI. This disconnect may also reduce ROI and prevent alignment between computational insights and actionable research outcomes.
Recommendation:
Pharmaceutical firms should adopt a phased integration approach, starting with pilot studies, and ensure that AI platforms are interoperable with existing infrastructure. Cross-functional collaboration between data scientists, clinicians, and operations teams can enhance adoption. Creating hybrid teams and change-management frameworks will facilitate smoother transitions and ensure AI systems are aligned with therapeutic goals.
3. Data Quality and Quantity
AI models require vast and clean datasets for accuracy. However, clinical and biological data can be incomplete, inconsistent, or fragmented across systems. Issues such as missing values, biased sampling, and variable data formats can reduce model robustness. Limited access to rare disease datasets also impairs generalizability and undercuts real-world performance in predictive tasks.
Recommendation:
To mitigate this, companies must invest in data curation and standardisation practices. Leveraging public repositories, consortia-driven datasets, and real-world evidence platforms can also improve volume and diversity. Techniques such as synthetic data generation or transfer learning can enhance model training, particularly in cases where data is rare or underrepresented.
4. Cost and Technical Expertise
AI initiatives require substantial investments in both financial and human capital. Recruiting and retaining AI talent, including data scientists, bioinformaticians, and computational biologists, can be challenging. Small and mid-sized firms may struggle to afford custom AI platforms, which can hinder scalability and delay deployment. Long development timelines can further strain limited R&D budgets.
Recommendation:
Strategic partnerships with academic labs or AI-focused biotech startups can reduce entry costs. Open-source frameworks and cloud-based tools also make AI more accessible. Upskilling existing scientific staff in AI fundamentals and offering cross-training programs can create in-house talent pipelines, reducing dependency on external consultants or vendors.
5. Lack of Standardisation
Inconsistent protocols across AI platforms—ranging from input formats to model evaluation metrics—limit reproducibility and interoperability. Without industry-wide standards, comparing models, validating outputs, and integrating tools into regulatory workflows becomes difficult. This creates ambiguity for developers, companies offering AI development services, and regulators alike, slowing the broader adoption of AI tools in drug pipelines.
Recommendation:
Efforts should focus on establishing shared ontologies, common validation frameworks, and standardised data exchange protocols. Participation in industry consortia, such as CDISC, the Pistoia Alliance, or the FDA’s AI initiatives, can promote harmonisation. Aligning AI development with regulatory guidance and publishing reproducible workflows enhances both scientific rigour and operational compatibility.
6. Interpretability and Transparency
Many AI models, particularly deep learning systems, operate as “black boxes,” providing limited insight into how predictions are made. In safety-critical fields, such as drug discovery, this lack of interpretability poses significant ethical and regulatory challenges. Researchers and regulators may hesitate to trust AI-driven conclusions without a clear rationale for the outputs.
Recommendation:
Explainable AI (XAI) techniques, such as SHAP values or attention mechanisms, can provide insights into model decision-making. Emphasising model transparency during development and using visual analytics dashboards helps stakeholders interpret predictions. Building models with interpretability by design and involving domain experts in validation processes enhances credibility and regulatory readiness.
7. Computational Resources and Time
AI training—especially for deep learning and generative models—requires significant computational power. Long training times, high GPU/TPU costs, and data transfer limitations can delay experimentation. For companies lacking dedicated infrastructure, scalability becomes a bottleneck. This may slow innovation or restrict AI applications to well-funded organisations.
Recommendation:
Cloud-based platforms, such as AWS SageMaker or Google Vertex AI, can offer scalable computing at manageable costs. Using pre-trained models or modular transfer learning approaches can cut down training time. Resource-efficient architectures (e.g., transformers, graph neural networks) and optimisation tools also reduce dependency on high-end hardware.
Future Directions and Trends of AI in Drug Discovery
Artificial intelligence technology is still evolving—and so is its role in the drug development process. These emerging trends highlight where the field is headed, from personalised medicine to integration with other frontier technologies, such as blockchain and synthetic biology.
1. Precision (personalised) medicine using AI
AI is enabling a shift toward precision medicine by analysing genetic, phenotypic, and clinical data to tailor treatments for individual patients. Machine learning models can predict how a specific drug will interact with a person’s unique biology. This reduces trial-and-error prescribing, improves therapeutic outcomes, and minimises side effects—especially in oncology, neurology, and rare disease treatment frameworks.
2. AI + blockchain + IoT integration
The convergence of AI, blockchain, and IoT is transforming the drug discovery ecosystem. AI interprets real-time IoT data from biosensors and devices, while blockchain secures data sharing and traceability. This trio enhances transparency in clinical trials, ensures the integrity of patient data, and enables decentralised trials. Together, they offer a more responsive, ethical, and data-driven approach to drug development.
3. Regulatory adaptation for AI models
Regulatory bodies are working to evolve guidelines that accommodate AI-based drug discovery systems. There is a growing focus on AI model validation, audit trails, and explainability. The FDA and EMA are exploring adaptive frameworks that can support dynamic models. This evolution is critical to ensure AI-generated insights meet safety, efficacy, and ethical standards while accelerating approvals for innovative therapies.
4. AI to address global health challenges
AI is increasingly applied to develop treatments for neglected tropical diseases, antimicrobial resistance, and pandemics. It helps identify repurposable drugs, optimise vaccine candidates, and simulate outbreak scenarios.
Open-source AI models and global collaborations are helping low- and middle-income countries leapfrog infrastructure barriers, ensuring more inclusive innovation in global drug discovery and development efforts.
5. LLMs in synthetic biology and biomanufacturing
Large Language Models (LLMs) are being trained on biological datasets to design proteins, optimise gene circuits, and generate synthetic DNA sequences. Their ability to understand and predict complex biological pathways accelerates the engineering of novel therapeutics and sustainable biomanufacturing processes. This integration marks a leap in AI’s role in designing living systems from scratch with programmable functions.
6. State Space Models and Multi‑omics Integration
Advanced modelling techniques, such as State Space Models (SSMs), enable AI to interpret time-series biological data, providing deeper insights into disease progression. When combined with multi-omics integration—genomics, proteomics, metabolomics—AI uncovers complex interactions driving diseases. These tools enhance target identification, stratify patient populations more accurately, and create more predictive drug development pipelines based on systems biology.
Partner with a Proven AI Development Team
Join leading pharma and biotech teams already using AI solutions to fast-track innovation and market success.
Conclusion
The impact of AI in drug development is already reshaping the pharmaceutical landscape, from discovery and design to clinical testing and market launch. By automating analysis, predicting outcomes, and uncovering biological insights more quickly than human researchers alone, AI is accelerating innovation while addressing long-standing inefficiencies.
Despite challenges in data privacy, interpretability, and regulatory compliance, the long-term promise is clear: faster drug pipelines, reduced R\&D costs, and more targeted, patient-centric therapies.
As AI models evolve and multi-disciplinary integration deepens—with technologies like blockchain, IoT, and LLMs—the future of drug development will be smarter, more connected, and more inclusive. Organisations that embrace AI now are not just improving their competitiveness—they’re shaping the future of global health.
In short, AI in drug development is not only transforming science but also transforming lives.
FAQs.
Exscientia and Sumitomo Dainippon used AI to create DSP-1181, a treatment for obsessive-compulsive disorder. It went from concept to clinical trials in under a year, which is crazy fast compared to traditional timelines. That project made headlines as one of the first true AI-designed drugs.
AI helps pinpoint the right molecules, match drugs to patient profiles, and predict side effects early. It makes drug treatment smarter, more personal, and faster. Instead of trial and error, AI provides researchers with solid leads, allowing them to focus on what’s most likely to work.
You’ll see machine learning, deep learning, neural networks, and natural language processing all working together. Each type tackles different problems—such as sorting data, identifying patterns in chemistry, or scanning medical journals for hidden connections. It’s a mix of brains working behind the scenes.
AI steps in from the start—helping pick targets, designing molecules, predicting toxicity, and even guiding clinical trials. It’s a super-powered research assistant that never sleeps. It speeds things up and reduces the number of failed experiments, which saves a significant amount of money and time.
Absolutely. AI can crunch years of research into weeks. It identifies patterns, screens compounds, and eliminates dead ends early. That means fewer wasted efforts and faster paths to clinical trials. Some drugs now hit testing phases in under 12 months, which is wild.
AI helps fine-tune aspects such as dosage, stability, and delivery method. It predicts how drugs behave in the body and identifies the optimal combinations for effective treatment. It’s used to create formulations that are more effective, longer-lasting, or easier to absorb—without relying so much on trial and error.
They dig through vast data sets—genetics, chemistry, and clinical records—and pull out connections that humans would miss. Think of them as ultra-fast pattern hunters that point scientists in the right direction, whether it’s finding new drug targets or predicting how a compound will behave.
Speed. AI can scan vast amounts of data to identify existing drugs that may be effective for new conditions. It’s way faster and cheaper than building something new from scratch. That’s especially handy in a crisis—like COVID—where time isn’t on your side.
Insilico Medicine used AI to develop a novel drug for pulmonary fibrosis. The entire preclinical design was completed in under 18 months—significantly shorter than usual. Their AI found targets, designed molecules, and optimised them. It’s a textbook case of how AI can fast-track drug pipelines.
AI still requires clean, high-quality data to function effectively—and that’s not always easy to obtain. Plus, some algorithms are black boxes, meaning it’s hard to explain why they make certain decisions. That lack of transparency can make regulators and researchers a little uneasy.
AI is used to analyse massive datasets, identify drug targets, design molecules, and predict how compounds will behave in the body. It helps researchers find promising leads faster and with greater accuracy, reducing the time and cost typically involved in early-stage drug discovery.
AI helps by identifying patterns in biological data, simulating compound interactions, and narrowing down candidates likely to be successful. It speeds up research, minimises failed experiments, and enables scientists to focus on the most viable options, cutting months—or even years—off traditional timelines.
Talk With Our Expert
USA
2102 Linden LN, Palatine, IL 60067
+1-708-515-4004
info@debutinfotech.com
UK
Debut Infotech Pvt Ltd
7 Pound Close, Yarnton, Oxfordshire, OX51QG
+44-770-304-0079
info@debutinfotech.com
Canada
Debut Infotech Pvt Ltd
326 Parkvale Drive, Kitchener, ON N2R1Y7
+1-708-515-4004
info@debutinfotech.com
INDIA
Debut Infotech Pvt Ltd
Sector 101-A, Plot No: I-42, IT City Rd, JLPL Industrial Area, Mohali, PB 140306
9888402396
info@debutinfotech.com




Talk With Our Expert
15+ years in IT
to deliver value that lasts
Over 500 success stories
including Disney, KFC, DocuSign & HDFC Bank
Team of 150 specialists
Web, mobile, Blockchain, AI & ML
Presence across 5 continents
Get Dedicated Account Managers operating in your time-zone.